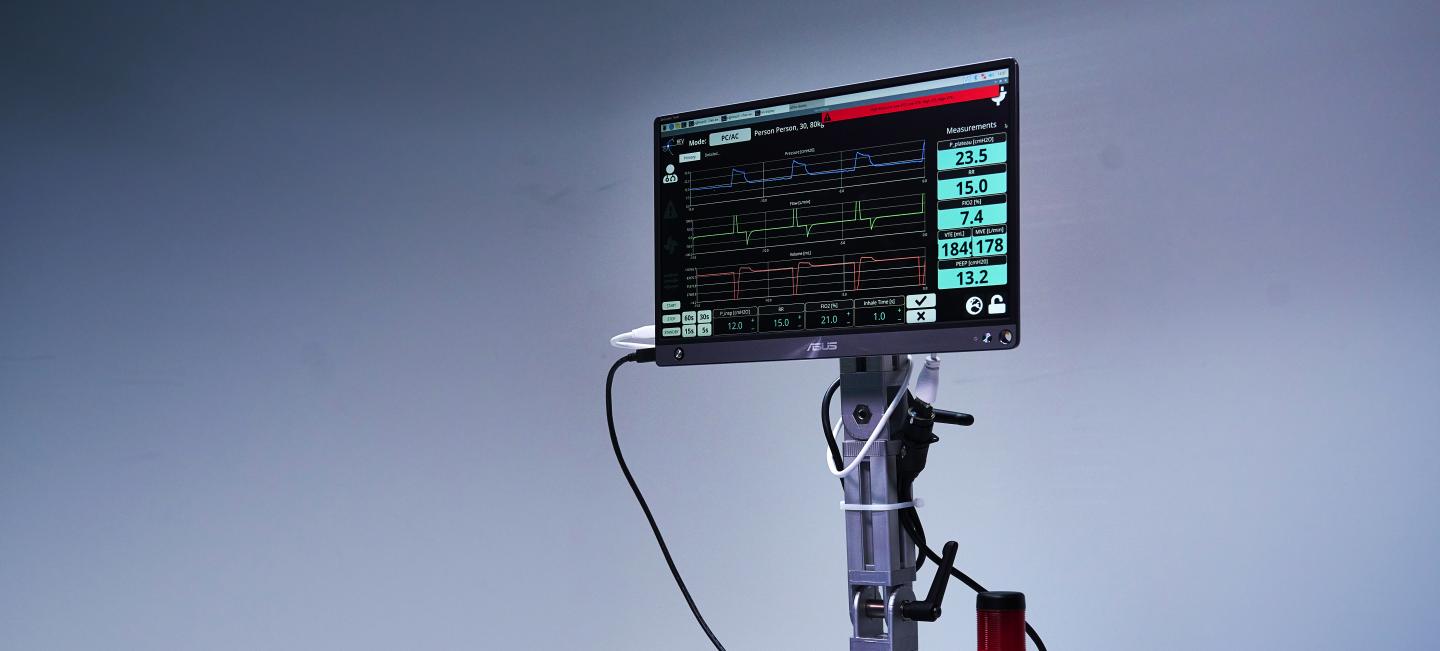
Ventilators provide oxygen enriched air to patients who have difficulties in breathing, or cannot breathe autonomously.
The High Energy Ventilator (HEV) project was born during the COVID-19 pandemic to design and prototype a versatile, high-quality, low-cost medical ventilator. It intends to provide long term alveolar ventilation support to patients, in or out of intensive care, for intubated and non-invasive cases. Clinical advice has guided the main choices during the design process, providing further insights to the COVID-19 official emergency guidelines from the MHRA, WHO and AAMI.
In 2020, UK Research and Innovation (UKRI), through the Global Challenges Research Fund, awarded funding to redesign the HEV for use in low- and middle-income countries. The High Performance Low Cost Ventilator (HPLV), based on the HEV prototype, relies less on compressed gases and mains electricity supply—making it more suitable for a variety of challenging settings in regions where optimal conditions might not be available.
The mechanical design of both the HPLV and HEV can provide a robust ventilator that is rapid and simple to construct with low cost, readily available components. Much attention is paid to fast response and precise and stable pressure delivery, simplification of ventilation modes, and an intuitive and familiar interface for clinicians. Functionality is aimed at the treatment of the vast majority of COVID-19 cases, freeing up the very high-end machines for the most intensive cases.
Both designs can provide standard Pressure Control and Pressure Support modes, as well as CPAP support. PEEP is provided for all modes, as is patient triggering for both the inhale and exhale phases of the breathing cycle. The pneumatic concept, i.e. ventilation provided via a low-pressure buffer, allows a precise and safe pressure control and accurate monitoring of flow rates. The step-down pressure design via the buffer puts safety up-front in the design.
The above features make the HEV and HPLV suitable for treatment of COVID-19 cases, and as general-purpose ventilators beyond COVID-19.
The ventilators may also be interesting for academic research, as a vehicle for implementing new ideas concerning ventilation.
(Video: CERN)
Advantages & Applications
Mechanical ventilation in hospitals (ICU and non-ICU), for intubated and non-invasive cases.
Specifications
- Functionality based on MHRA, WHO and AAMI guidelines for COVID-19 emergency ventilators.
- Low-cost design based on commercially available components, thanks to the two-step pneumatic design.
- Design inherently flexible and modular, for adaption to different requirements and environments.
- High quality breath control and breath support, with patient comfort set as a priority.
- Air/Oxygen mixing provided internally, no need for an external unit.
- Intuitive and ergonomic touch-screen control.
- Equipped with standard bulkhead thread connector, for easy adaption to match hospital connectors around the world.
- Can be powered by a standard AC connection, or a 24V DC source from a UPS backup.
- Internal battery provides up to 45 minutes autonomy, can be augmented with a second external battery
Company Selection Criteria
Any organisation applying for a licence to commercially exploit the HPLV will be asked to provide documentation on the basis of the expectations and requirements detailed below. CERN reserves the right to ask for additional information where such a request would facilitate taking a decision on a licence.
All licensing decisions will be made at CERN’s sole discretion, and will not be subject to appeal.
Essential
- A development and commercialisation plan to supply devices for use in one or more DAC-list countries.
- Evidence of an understanding of the medical device regulatory, certification and risk management process, including the provision of: a draft plan for attaining certification of the HPLV in target market(s); a draft risk management plan outlining the main risk categories; and a draft plan for implementing a quality management system (QMS) for the large-scale manufacture of the HPLV, according to ISO 13485.
- Each company that is successful in receiving a licence for the HPLV is expected to undertake their own full risk analyses and implement their own QMS for manufacturing the HPLV, and is expected to obtain any necessary certification or regulatory approval(s) for use or distribution of the HPLV, within the territory, or territories, in which the use will take place.
- Evidence of manufacturing (either directly or through appropriate subcontracting partners) similarly-complex devices and/or systems (not necessarily medical) that include mechanical hardware, sensors, electronics, controls, and software.
- Minimal technical support needed from CERN and/or the other HPLV partners.
- A common forum for licensees will be provided, in which questions can be asked. The level of support provided through this forum will be solely at CERN’s and/or the other HPLV partners’ discretion.
- Licensees may request additional consultancy from any of the HPLV partners, but its provision is solely at the discretion of the partner that receives such request.
- Acceptance of the non-negotiable licence terms.
Desirable
- No technical support needed from CERN and/or the other HPLV partners.
- Currently a medical device manufacturer.
- Can manufacture devices locally in the target market(s).
- Evidence of previous collaboration with the medical community in target market(s).
- Evidence of an existing QMS system(s) for manufacturing similarly-complex devices, preferably a certified QMS according to ISO 13485.